 The University of Arizona BIO5 Institute has allocated more than $70,000 in funding to a multi-disciplinary research team led by UA College of Engineering’s Eleonora Tubaldi, PhD, and UA College of Medicine – Tucson’s Diego Celdran Bonafonte, DVM, PhD.
The University of Arizona BIO5 Institute has allocated more than $70,000 in funding to a multi-disciplinary research team led by UA College of Engineering’s Eleonora Tubaldi, PhD, and UA College of Medicine – Tucson’s Diego Celdran Bonafonte, DVM, PhD.
Dr. Bonafonte is a BIO5 member, assistant professor in the UA Division of Nephrology and part of the Arizona Kidney and Vascular Program (AKVP) led by Prabir Roy-Chaudhury, MD, PhD, division chief and program director, to improve imaging analyses of blood flow within a vascular access point that allows patients on hemodialysis to be connected to the dialysis machine which cleans their blood.
Defining the Problem
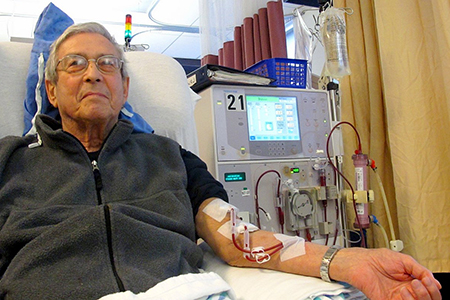
The reason for this grant is that arteriovenous fistulas (ATF)—a common type of vascular access point surgically created in a vein, often at the crook of a person’s arm, to draw and return a patient’s blood after it’s treated—often wear out. That’s due to blood vessel wall remodeling that results in scarring and vasoconstriction.
“There are currently over 450,000 patients in this country on hemodialysis, which unfortunately has a very poor survival rate (with 20 percent mortality in the first year and, by year 5, only 41 percent remain alive),” noted Dr. Roy-Chaudhury, who also is co-chair of the Kidney Health Initiative, a public-private partnership of the American Society of Nephrology and U.S. Food and Drug Administration. “Patient survival is worse than for the vast majority of cancers, except lung and brain cancer. Vascular access dysfunction is an important contributor to this mortality—and that is why this work is so important.”
Eleonara’s strong expertise in fluid mechanics, soft tissue mechanics and fluid-solid interaction in both numerical and analytical modeling make her an ideal investigator to provide the required engineering skills to the project, he added.
A Grant for Hope
The research team hopes ultimately to develop new protocols for a better, open access, less complex, less expensive software application that provides computational flow dynamic (CFD) analyses of magnetic resonance imaging (MRI) technologies that would lead to advances to allow the AVF to last longer, thus reducing patient morbidity and mortality for those suffering from chronic kidney disease (CKD) or end-stage renal disease (ESRD).
“Congratulations! I am pleased to inform you that your BIO5 Team Scholars Program proposal, ‘MRI-to-CFD Pipeline for Hemodynmic Profiling of Murine Arteriovenous Fistula’ has been recommended for funding,” noted BIO5 Director Jennifer K. Barton, PhD, in the award letter.
Principal investigator on the research team, Dr. Tubaldi, upon informing Dr. Roy-Chaudhury of the funding approval, said she was “looking forward to continuing our fruitful collaboration!”
Among other grants Dr. Tubaldi is collaborating with Dr. Roy-Chaudhury on is one he won as a principal investigator from the National Institute of Diabetes, Digestive and Kidney Diseases, a unit of the National Institutes of Health (NIH), in March 2017 for $275,529 to help Inovasc LLC develop “HELical Biodegradable Photochemical (HELP) Stents for AVF Maturation.”* In September 2017, he also won a grant for $603,715 from the National Institute on Aging, another NIH unit, for a study with Cylerus Inc. on “Localized Delivery of Sirolimus to Hemodialysis Vascular Access Grafts” that is focused on reducing incidence of infection at the vascular access point.**
About the Research Team
 Eleonora Tubaldi, PhD – a faculty member in the Department of Aerospace and Mechanical Engineering, brings to the team her expansive experience on nonlinear dynamics of fluid-vessel interactions when exposed to pulsed flow. She will play a key role in the team by developing an innovative approach to the study and analysis of AVF’s CFD by developing a MRI-to-CFD protocol using the open-source pipeline software SimVascular, Dr. Roy-Chaudhury said that this proposal brings out the importance of multidisciplinary collaborations which this University is all about and which is the only way to develop new treatments that help patients. If you had told me when I graduated from medical school that I would be working with an aeropace engineer I would never have believed you but that’s the way we are going to solve difficult clinical problems. I particularly want to recognize the vision and the foresight of Dr Barton and the BIO5 leadership team in supporting such innovative projects cross cutting projects.
Eleonora Tubaldi, PhD – a faculty member in the Department of Aerospace and Mechanical Engineering, brings to the team her expansive experience on nonlinear dynamics of fluid-vessel interactions when exposed to pulsed flow. She will play a key role in the team by developing an innovative approach to the study and analysis of AVF’s CFD by developing a MRI-to-CFD protocol using the open-source pipeline software SimVascular, Dr. Roy-Chaudhury said that this proposal brings out the importance of multidisciplinary collaborations which this University is all about and which is the only way to develop new treatments that help patients. If you had told me when I graduated from medical school that I would be working with an aeropace engineer I would never have believed you but that’s the way we are going to solve difficult clinical problems. I particularly want to recognize the vision and the foresight of Dr Barton and the BIO5 leadership team in supporting such innovative projects cross cutting projects.
 Diego Celdran-Bonafonte DVM, PhD – research assistant professor, College of Medicine, Department of Medicine. As a member of the Division of Nephrology and AKVP, Dr. Celdran has experience in the development and optimization of animal models and is currently involved as a co-investigator in multiple research grants (National Institutes of Health, Veterans Administration) involving the use of animal models to improve the outcomes of all the main forms of vascular access for dialysis. A veterinarian by training, he brings in his extensive expertise on animal surgery, uremic vascular biology and the creation and follow-up of murine vascular access models. The AKVP has experience in induction of kidney failure on murine models as well as the creation, clinical follow-up (high definition ultrasound, micro computed tomography—or micro CT) and laboratorial assessment (histomorphometrics, gene expression studies) of murine AVFs.
Diego Celdran-Bonafonte DVM, PhD – research assistant professor, College of Medicine, Department of Medicine. As a member of the Division of Nephrology and AKVP, Dr. Celdran has experience in the development and optimization of animal models and is currently involved as a co-investigator in multiple research grants (National Institutes of Health, Veterans Administration) involving the use of animal models to improve the outcomes of all the main forms of vascular access for dialysis. A veterinarian by training, he brings in his extensive expertise on animal surgery, uremic vascular biology and the creation and follow-up of murine vascular access models. The AKVP has experience in induction of kidney failure on murine models as well as the creation, clinical follow-up (high definition ultrasound, micro computed tomography—or micro CT) and laboratorial assessment (histomorphometrics, gene expression studies) of murine AVFs.
 Jose Rosado-Toro, PhD – postdoctoral fellow, College of Medicine, Department of Medical Imaging. Dr. Rosado possesses an extensive expertise on image analysis and segmentation. As part of his doctoral work he developed a novel segmentation technique that extended use of polar dynamic programming to segment complex shapes that is patent-pending. During this last year, he has been working with Drs. Celdran-Bonafonte and Roy-Chaudhury in incorporating and modifying an MRI-to-CFD protocol for swine AVFs. He has used his knowledge of MR image acquisition protocols to help generate accurate CFDs by incorporating geometric information that identifies and spatially locates the site of the flow image in respect to the MRI black blood sequences. The skills he has acquired during this year will translate seamlessly to the creation of an MRI-to-CFD protocol for murine models.
Jose Rosado-Toro, PhD – postdoctoral fellow, College of Medicine, Department of Medical Imaging. Dr. Rosado possesses an extensive expertise on image analysis and segmentation. As part of his doctoral work he developed a novel segmentation technique that extended use of polar dynamic programming to segment complex shapes that is patent-pending. During this last year, he has been working with Drs. Celdran-Bonafonte and Roy-Chaudhury in incorporating and modifying an MRI-to-CFD protocol for swine AVFs. He has used his knowledge of MR image acquisition protocols to help generate accurate CFDs by incorporating geometric information that identifies and spatially locates the site of the flow image in respect to the MRI black blood sequences. The skills he has acquired during this year will translate seamlessly to the creation of an MRI-to-CFD protocol for murine models.
The research team hopes to leverage the information they gain from this research to apply for additional funding to broaden their research goals.
In their BIO5 funding proposal, the research team—which will operate from lab space provided by the BIO5 Institute—wrote: “Chronic kidney diseases is a heterogeneous disease that progressively reduces the function and structure of the kidney, leading to an End-Stage Renal Disease (ESRD) phase where kidney transplant or dialysis is required. Dialysis vascular access is the ‘lifeline’ for well over 400,000 patients on hemodialysis in the United States, since the ability to be connected to the dialysis machine relies on the presence of a functional vascular access. Due to the many complications associated with all three forms of such access (i.e., AVFs—the preferred mode of dialysis, dialysis grafts and tunneled dialysis catheters [TDCs]), vascular access is the ‘Achilles heel’ of hemodialysis, resulting in a very significant morbidity and mortality that translates into a huge economic burden.”
* The research is supported by the NIDDK under Award No. 1R41DK111332-01. The content solely is the responsibility of the authors and does not necessarily represent the official views of the NIH.
** The research is supported by the NOA under Award No. 1R44AG059279-01A1. The content solely is the responsibility of the authors and does not necessarily represent the official views of the NIH.
ALSO SEE:
“Fall Photo Galleries Showcase Busy Agenda for UA Department of Medicine in 2017” (see “Nephrology Research Grants Aid in New, Safer Alternatives for Dialysis”) | Posted Dec. 19, 2017

