If less is more, is more better? Both may be true (or not) for two University of Arizona Health Sciences researchers studying the impact of redox chemical reactions—specifically reductive stress—and metabolism in pulmonary hypertension and the risks for cardiovascular disease. The final answers may depend on your gender.
 Early last fall, the University of Arizona husband-and-wife investigator team of Olga Rafikova, MD, PhD, and Ruslan Rafikov, PhD, (pictured above and left) assistant professors at the UA College of Medicine – Tucson, won two five-year R01 awards from the National Heart, Lung and Blood Institute, a unit of the National Institutes of Health.
Early last fall, the University of Arizona husband-and-wife investigator team of Olga Rafikova, MD, PhD, and Ruslan Rafikov, PhD, (pictured above and left) assistant professors at the UA College of Medicine – Tucson, won two five-year R01 awards from the National Heart, Lung and Blood Institute, a unit of the National Institutes of Health.
Drs. Ruslan Rafikov and Olga Rafikova work with lab techs Elissa Williams and Matt McBride in their new laboratory space in the UA Life Sciences North building.
The grants, valued at about $4 million—or nearly $1.92 million each—are to study pathogenic mechanisms involving molecular and metabolic pathways influencing pulmonary arterial hypertension (PAH), a form of high blood pressure in the lungs that leads to an enlarged heart and eventual heart failure.
The two researchers in the UA Division of Translational and Regenerative Medicine work with pulmonologists, cardiologists and critical care, biostatisticians and proteomic specialists within the UA Department of Medicine, the largest department in the UA College of Medicine - Tucson.
Congratulating the pair, Jason X.-J. Yuan, MD, PhD, a professor  of medicine and physiology, division chief and associate vice president for translational medicine, UA Health Sciences, noted that PAH is a fatal and progressive disease that predominantly affects women.
of medicine and physiology, division chief and associate vice president for translational medicine, UA Health Sciences, noted that PAH is a fatal and progressive disease that predominantly affects women.
“Studies on lung vascular pathobiology and pulmonary vascular disease are one of the major research focuses in the Division,” Dr. Yuan said. “Drs. Rafikova and Rafikov’s new grants provide an additional opportunity for them and other investigators to develop new research projects that emphasize identifying drug targets and ultimately developing novel therapeutic approaches for pulmonary arterial hypertension.”
Her project is titled, “HMGB1 and Gender Difference in Pulmonary Arterial Hypertension,” supported by NHLBI under award number 1R01HL133085-01. His is titled, “Anaplerotic Reprogramming of Endothelial Cells in Pulmonary Hypertension,” supported by NHLBI under award number 1R01HL132918-01.
Dr. Rafikova’s work focuses on gender disparities in the function of genetic pathways governing PAH, also known simply as pulmonary hypertension or PH. Dr. Rafikov’s focuses on how metabolic processes influence development and progression of PAH. In this context, both researchers are studying reductive and oxidative (redox) stress mechanisms and ways to control the disease on a molecular or metabolic level to determine therapies that might be developed to ease or cure related health complications.
The Problem
 Chest radiograph overlaid with illustration showing pulmonary circulation [Wikimedia].
Chest radiograph overlaid with illustration showing pulmonary circulation [Wikimedia].
PAH is a form of high blood pressure that affects the right side of the heart and pulmonary arteries that supply blood to the lungs.
For people suffering from it, damage to blood vessels that carry blood from your heart to your lungs causes the arterial walls to thicken and harden. Narrower passageways increase blood pressure and make the right ventricle of the heart work harder to pump blood. This overload eventually induces right ventricular hypertrophy, or an enlarged heart. Over time, the heart weakens. That leads to heart failure.
PAH is a rare but deadly disease, affecting an estimated 15-50 cases per million people. This can be higher in at-risk groups such as HIV-infected patients, those with systemic sclerosis or sickle cell disease. Symptoms include shortness of breath during routine activity, tiredness, chest pain, a racing heartbeat, upper right stomach pain and decreased appetite. There is no cure, but treatment can help control symptoms.
Inevitably fatal, PAH has a very poor prognosis left undiagnosed and untreated with survival rates between six years in its mildest form or as low as six months for the most advanced stage. Tests and procedures in diagnosing the disease include lung function tests, echocardiograms, chest X-rays, CT scans or MRIs, electrocardiograms (EKGs), and right heart catheterization.
Her Approach
Last fall, Dr. Rafikova was an invited speaker at the Society for Redox Biology and Medicine annual meeting in Boston where she discussed gender differences in effects of antioxidant diets in management of cardiovascular disease. Referring to a connection between oxidative-reductive balance in PAH for each gender, she noted a shift toward a more reduced state for men contributes to progression of chronic disease by promoting arterial and cardiac inflammation and fibrosis—scarring and thickening—that leads to PAH/PH, an enlarged heart and heart failure.
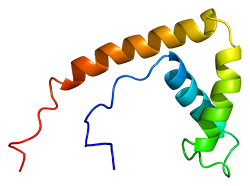 Her grant project delves deeper, looking at the role of high-mobility group box 1 (HMGB1) proteins—pictured right [Wikimedia]—and differences in gender response in PAH. HMGB1 is an important chromatin protein secreted by immune cells that also has been described recently as a damage-associated molecular pattern (DAMP) molecule in that it serves as a “danger signal” when released from dying cells—including those damaged by high blood pressure in PAH—depending upon how they die.
Her grant project delves deeper, looking at the role of high-mobility group box 1 (HMGB1) proteins—pictured right [Wikimedia]—and differences in gender response in PAH. HMGB1 is an important chromatin protein secreted by immune cells that also has been described recently as a damage-associated molecular pattern (DAMP) molecule in that it serves as a “danger signal” when released from dying cells—including those damaged by high blood pressure in PAH—depending upon how they die.
Apoptosis and necrosis are two main types of cell death. Apoptosis is a natural, programmed cell death process that allows undamaged cells to utilize building blocks from the dying cell and doesn’t induce activation of the immune system. In contrast, necrosis occurs due to injury or damage of cell membrane integrity, which results in a passive release of the cell content outside the cell, attracting immuno-defense cells and initiation of inflammation. HMGB1 is released in a passive manner and a reduced state, if a cell dies by necrosis; and gets actively secreted in oxidized form during apoptotic cell death.
A recently discovered gender difference in the type of cell death that may occur in response to initial damage from PAH indicates males have a higher chance for their cells to die by necrosis and females by apoptosis. In her research, Dr. Rafikova notes the different forms of HMGB1 (reduced or oxidized) may initiate completely different molecular pathways. This may explain why women have a higher susceptibility to PAH, but men have a much lower survival rate and are more likely to suffer from pulmonary wall inflammation and right ventricular fibrosis.
“Males and females seems to be much more different than we have always expected,” Dr. Rafikova said. “The gender difference has been discussed for years. However, in clinical practice men and women are getting exactly the same treatments, which may explain why despite the effort, we still have unmet therapeutic needs in treatment of many diseases. PAH has a very strong gender dimorphism, but the particular pathways involved in manifestation of these disparities were not clear. I believe we have uncovered the important mechanisms involved in developing a pro-inflammatory phenotype in males and proliferative phenotype in females.”
The solution, she feels, are specially designed peptides—short chains of amino acid monomers linked by chemical bonds. Differently targeted peptides would specifically block inflammation in males—initiated upon binding of the reduced-state HMGB1 protein from necrotic cells to pro-inflammatory toll-like receptor 4 (TLR4) proteins. Or they would block activation of over-proliferation seen in females—by preventing interaction of apoptotic-cell-released, oxidized-state HMGB1 proteins with the protein for receptors for advanced glycation endproducts (RAGE). TLR4 is responsible for activating the body’s innate immune system. RAGE is a pattern recognition receptor thought to play a similar binding role in signaling cascades and gene expression that leads to increased proliferation and adhesion of impaired cells in PAH.
“These unique peptides were not only designed to specifically investigate the contribution of inflammation and proliferation in development of PAH in either gender, but have a great potential in becoming the first gender-specific therapeutic intervention for PAH, and beyond,” Dr. Rafikova said.
His Approach
Dr. Rafikov’s project takes a different tack, studying the pathogenic role of reactive oxygen species (ROS) and the Akt/mTOR signaling cascade— both intracellular signaling pathways important in regulating the cell cycle—in development and progression of pulmonary hypertension.
He is looking at metabolic reprogramming of a thin layer of cells that line the interior surface of blood vessels and lymphatic vessels known as endothelial cells via manipulation of anaplerotic reactions to better control the disease and its progression.
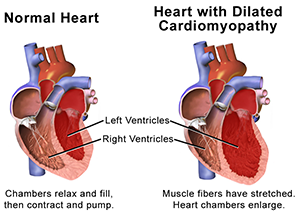 Anaplerotic reactions are metabolic pathways that allow synthesis of complex molecules in living organisms from simpler ones together with the storage and release of energy. This anabolic biosynthesis in the lung cells leads to cancer-like growth, or proliferation, of pulmonary vasculature that leads in turn to thickening of arterial walls and increased pulmonary vascular resistance (PVR). In other words, the narrowing and hardening of arteries increases pressure needed for blood flow. In the heart, it also contributes to an enlarged right ventricle—pictured right [Wikimedia]. That results in a weaker heart, heart failure and, ultimately, patient death.
Anaplerotic reactions are metabolic pathways that allow synthesis of complex molecules in living organisms from simpler ones together with the storage and release of energy. This anabolic biosynthesis in the lung cells leads to cancer-like growth, or proliferation, of pulmonary vasculature that leads in turn to thickening of arterial walls and increased pulmonary vascular resistance (PVR). In other words, the narrowing and hardening of arteries increases pressure needed for blood flow. In the heart, it also contributes to an enlarged right ventricle—pictured right [Wikimedia]. That results in a weaker heart, heart failure and, ultimately, patient death.
Metabolic reprogramming of lung cells in PH is currently a hot area of study. It was found that vascular cells undergo glycolytic switch—a so-called Warburg effect related to how cancer cells synthesize energy. Utilizing glucose for production of adenosine triphosphate (ATP)—which is used in cells as a coenzyme in intracellular energy transfer—rather than effective production through mitochondrial oxidative phosphorylation (known as the Krebs cycle) is now well described for the pathogenesis of PH, Dr. Rafikov said. His research focuses on the next step beyond the Warburg effect to explain how metabolism switches to adapt to the demand of growing or proliferating cells and to stop that growth.
“Basically, when cells have normal function, they generate power from the mitochondria—a cell’s energy factory—using ATP. But when cells become cancer-like and proliferate too fast as in pulmonary hypertension, they do not depend on the mitochondria. They choose glucose—or sugar—to generate power,” Dr. Rafikov said. Since that didn’t fully account for the rapid cell proliferation, he took his analysis further to determine that the mitochondria had shifted from generating power to generating cellular building blocks that supplied the substrate for the growing cells via anaplerotic reactions.
 In spite of this obvious relevance of anaplerotic pathways in PH, it has not been characterized before, Dr. Rafikov said. His grant will study pyruvate carboxylation, which is an anaplerotic reaction he feels is important in the mitochondria’s switch from being the cell’s powerhouse to building block production that leads to cell proliferation in PH.
In spite of this obvious relevance of anaplerotic pathways in PH, it has not been characterized before, Dr. Rafikov said. His grant will study pyruvate carboxylation, which is an anaplerotic reaction he feels is important in the mitochondria’s switch from being the cell’s powerhouse to building block production that leads to cell proliferation in PH.
“We found that the enzyme pyruvate carboxylase (pictured left in tetrameric image) is upregulated and activated in the pulmonary arteries in our different pre-clinical models of PH,” Dr. Rafikov explained. “This is the first evidence that anaplerosis can be attributed to metabolic changes in the lung and heart. Our attempt to inhibit pyruvate carboxylase produced nice results in that pre-clinical model. So, I think, our first step is to regulate metabolism in order to fight with the pulmonary hypertension as would be the case with any other commonly considered ‘metabolic’ diseases such as diabetes mellitus.”
He feels if he could resolve the issue of metabolic pathways and their alteration to influence cell proliferation, he could better understand how to treat PH and other metabolic-related diseases such as diabetes, cancer, etc.
Personalized Medical Solutions
In both Drs. Rafikova’s and Rafikov’s work there lie the roots of precision medicine solutions tailored for the genetic, redox or metabolic conditions of the patient, both of these investigators acknowledge.
For her part, this may vary based on a patient’s gender or whether they are in early or late stages of PAH/PH, Dr. Rafikova added.
“What we are trying to identify are specific biomarkers to help us determine which phenotype of the disease each particular patient has,” she said. “This redox state is a small step in the direction. It helps us determine the different types of disease that we can separate out for treatment. In the future, we’ll really want to find more markers that will help us identify particular types of disease in each patient—and tailor they’re treatment to those specific needs. We have already initiated a precise search of such markers with great help from Dr. Mandarino and Dr. Langlais and their proteomics team here.”
 Larry Mandarino, PhD, is a UA professor of medicine and chief of the Division of Endocrinology, Metabolism and Diabetes, and Paul Langlais, PhD, is an assistant professor of biochemistry/molecular biology affiliated with the UA Center for Disparities in Diabetes, Obesity and Metabolism—of which Dr. Mandarino is also director.
Larry Mandarino, PhD, is a UA professor of medicine and chief of the Division of Endocrinology, Metabolism and Diabetes, and Paul Langlais, PhD, is an assistant professor of biochemistry/molecular biology affiliated with the UA Center for Disparities in Diabetes, Obesity and Metabolism—of which Dr. Mandarino is also director.
In his research, Dr. Rafikov said the same goals hold for him on determining different treatments related to specific metabolic conditions of the patient.
“That’s kind of, for me, personalized medicine,” he said.
First, though, come pre-clinical and clinical trials to test and refine any therapies before you’ll see any treatments in your doctor’s office or supplements on store shelves.
EXTRA INFO: Good, Bad & Antimitogenic
To learn more about Drs. Olga Rafikova’s and Ruslan Rafikov’s research, see the following articles:
- “Estrogen: Good, Bad, or Both?” - Hypertension. 2014 Mar;63(3):449-50. doi: 10.1161/HYPERTENSIONAHA.113.02500. Epub 2013 Dec 23. PMCID: PMC3945415
- “2-Ethoxyestradiol is antimitogenic and attenuates monocrotaline-induced pulmonary hypertension and vascular remodeling” – Vascul Pharmacol. 2008 Apr-Jun;48(4-6):174-83. doi: 10.1016/j.vph.2008.02.001. Epub 2008 Feb 13. PMID: 18373958
ALSO SEE:
“To Supplement or Not: A Role for Antioxidant Vitamins in the Management of Heart Failure?” | American Journal of Medicine, Posted August 2016
“UA Researchers Add New Twist in Debate over Free Radicals, Antioxidants in Cardiovascular Health — Less Isn’t Necessarily More” | DOM News, Posted Nov. 17, 2015


