Dr. Clara Curiel’s research interests are in photobiology, early skin cancer detection, biomarker development, and primary prevention. She has taken the initiative to address important clinical gaps in the skin cancer prevention field through innovative clinical study designs, and effective execution of clinical studies. She has served as a lead dermatologist in eight clinical studies and as a PI in seven clinical trials (4 pilot, 3 Phase 1, and 3 Phase 2 studies, 1 phase 3 study) as part of the UA NCI/DCP Cancer Chemoprevention Consortium Agreement, PO1 Skin Cancer Prevention studies, and sponsored studies.
 Dr. Curiel’s clinical experience in skin cancer is highlighted by the completion of a fellowship in skin photobiology at Wellman Laboratories of Photomedicine at Boston’s Massachusetts General Hospital and a tumor biology fellowship at The Ludwig Boltzman Institute in Germany, followed by a residency training in Dermatology at the combined Boston University /TUFTS program and a skin cancer clinical fellowship. Subsequently, she has successfully executed a leading role over the past 16 years as a co-director of two Multidisciplinary Cutaneous Oncology Programs (COP) (BIDMC/HMS and UACC), as a director of two specialized Pigmented Lesion Clinics (PLC)—BIDMC/HMS and UACC, and most recently as the clinical director of the University of Arizona Skin Cancer Institute.
Dr. Curiel’s clinical experience in skin cancer is highlighted by the completion of a fellowship in skin photobiology at Wellman Laboratories of Photomedicine at Boston’s Massachusetts General Hospital and a tumor biology fellowship at The Ludwig Boltzman Institute in Germany, followed by a residency training in Dermatology at the combined Boston University /TUFTS program and a skin cancer clinical fellowship. Subsequently, she has successfully executed a leading role over the past 16 years as a co-director of two Multidisciplinary Cutaneous Oncology Programs (COP) (BIDMC/HMS and UACC), as a director of two specialized Pigmented Lesion Clinics (PLC)—BIDMC/HMS and UACC, and most recently as the clinical director of the University of Arizona Skin Cancer Institute.
Both the COP and PLC clinical activities have been integrated with translational research studies, making them a unique setting for high-quality patient care and a critical resource for accrual into clinical studies and establishment of patient registries and tissue bank. Through local and national collaborations with colleagues leading similar efforts, we are able to identify and address as dermatologists critical gaps in the skin cancer field through multi-institutional studies, consensus and opinion statements to guide future investigations.
Within the area of skin cancer prevention, she has extensively lead and participated in the development and execution of epidemiological and interventional studies, many of them specifically related to skin cancer chemoprevention studies. She lead the first case-control study with over 400 cases melanoma at the Harvard Cancer Center indicating a potential role of NSAIDs as a preventive agent. This effort was followed by the first NCI/DCP funded interventional study to evaluate the safety and tolerability of Sulindac in high-risk nevi phenotypes at risk for melanoma development. She is also one of the founder dermatologists of the Oncology Cooperative/Melanoma Prevention Working Group in 2002 seeking to enhance a multidisciplinary approach nation-wide to the increasing need for skin cancer prevention. Dr. Curiel has served as a Co-PI on the previously funded Skin Cancer Chemoprevention Program Project Grant at the UACC, and effectively lead the completion of the proposed translational studies.
In addition to leading the above mentioned epidemiological and clinical studies, her involvement in the identification and validation of biomarkers and molecular technologies that can be effectively implemented in experimental and clinical studies has been an important focus of her work. Surrogate indicators of disease progression and targeted therapy have been proven to be critical in the effective and innovative design of clinical studies in the skin cancer filed.
Another one of her research focuses includes the implementation of imaging and other non-invasive technologies to enhance early skin cancer detection and implementation in experimental and clinical studies. 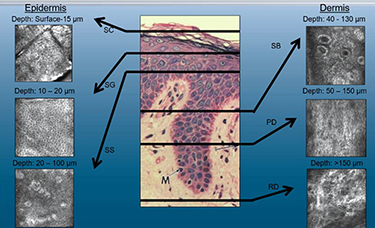 In many instances, interventional prevention studies in skin cancer are strengthen by the combination of novel non-invasive monitoring tools coupled with relevant biomarker assessment. Specifically, she has been engaged in the use of in vivo reflectance confocal microscopy (RCM), optical coherence tomography, epiluminescence microscopy, and spectropolarimetry within the skin cancer field. Most recently, her Program Project Grant team acquired a multilaser RCM instrument for implementation in ongoing actinic keratoses (AKs) studies. They are also in the process of standardizing RCM criteria for the long-term evaluation of photodamaged skin and AKs. Dr. Curiel’s tam is also in the process of establishing a photoacoustic clinical prototype for assessment of cutaneous malignancies and other skin conditions.
In many instances, interventional prevention studies in skin cancer are strengthen by the combination of novel non-invasive monitoring tools coupled with relevant biomarker assessment. Specifically, she has been engaged in the use of in vivo reflectance confocal microscopy (RCM), optical coherence tomography, epiluminescence microscopy, and spectropolarimetry within the skin cancer field. Most recently, her Program Project Grant team acquired a multilaser RCM instrument for implementation in ongoing actinic keratoses (AKs) studies. They are also in the process of standardizing RCM criteria for the long-term evaluation of photodamaged skin and AKs. Dr. Curiel’s tam is also in the process of establishing a photoacoustic clinical prototype for assessment of cutaneous malignancies and other skin conditions.
An additional aspect of her interest in digital imaging is reflected by her leadership role within the International Skin Imaging Collaboration where she co-leads the imaging acquisition working group. The overall objective of this expert group is to lead the standardization of digital imaging in the dermatology field.
Her ongoing research projects include the following:
- Investigation of immune checkpoint expression in cutaneous squamous cell carcinoma. Ongoing
- Pilot study on the signaling pathway modulation effect in the skin of patients undergoing treatment by checkpoint inhibitors. Ongoing
- Modulation of targeted pathways in the skin by acute solar simulated light in healthy volunteers. Ongoing
- Implementation of in vivo reflectance confocal microscopy in dermatological conditions. Ongoing
- Understanding of barriers for implementation of standards in imaging acquisition recommendations. Ongoing
- Dysplastic nevi and risk of malignant transformation. Analysis underway
- Understanding the effect of emollient and natural course of actinic keratoses. A randomized study implementing in vivo confocal microscopy and biomarker studies. Analysis underway
- A Prospective Observational Study of Treatment Patterns and Effectiveness and Safety Outcomes in Advanced Basal Cell Carcinoma and Basal Cell Carcinoma Nevus Syndrome Patients. Analysis underway
- A Single Arm Phase 2 Study of talimogene laherparepvec (T-VEC) in patients with cutaneous squamous cell cancer. Expected to open for accrual 1/2018.


