 Over the last decade, my National Institutes of Health (NIH)-funded laboratory has been a leader in studying the Pimproteinkinase (Pim). In a lymphoma model, Pim was discovered as a protein kinase that enhanced the ability of the c-Myc gene to induce lymphomas.
Over the last decade, my National Institutes of Health (NIH)-funded laboratory has been a leader in studying the Pimproteinkinase (Pim). In a lymphoma model, Pim was discovered as a protein kinase that enhanced the ability of the c-Myc gene to induce lymphomas.
We were the first to demonstrate that the levels of Pim protein kinase were regulated by hematopoietic growth factors, and prevented the apoptotic death of these cells. We explained this result in part by demonstrating that the proapoptotic BH3 protein, BAD, was a Pim substrate and that phosphorylation of BAD inhibited its ability to regulate apoptosis.
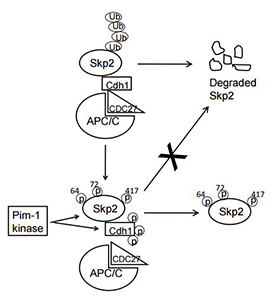 This substrate has been used by Biopharma to screen and produce small molecule Pim inhibitors that are now in human clinical trials as anticancer agents. In addition, the Kraft laboratory has produced their own small molecule Pim inhibitor, and this agent was shown to enhance apoptosis by clinical inhibitors of the Bcl-2 family of proteins.
This substrate has been used by Biopharma to screen and produce small molecule Pim inhibitors that are now in human clinical trials as anticancer agents. In addition, the Kraft laboratory has produced their own small molecule Pim inhibitor, and this agent was shown to enhance apoptosis by clinical inhibitors of the Bcl-2 family of proteins.- Using triple knock-out fibroblasts containing none of the 3 Pim kinase family members, we demonstrated that cells in the absence of Pim grow very slowly and this slow growth can be overcome by the expression of c-Myc.
- Over expression of Pim enhances the growth of human prostate cancer. This observation is shown to be in part regulated by phosphorylation and activation of mTOR signal transduction pathway and most recently by the phosphorylation of eIF-4B, a protein that regulates translation by modifying eIF-4A activation. Because AKT is highly activated in prostate cancer secondary to PTEN loss, the role of Pim was investigated when prostate tumor cells were treated with inhibitors of the AKT kinase. Pim was found to enhance the translation of mRNAs encoding tyrosine kinases by a mechanism involving 5’ IRES sequences.
- Preclinical studies are underway with Novartis to validate the combination of a PI3K and Pim inhibitor for the treatment of prostate cancer.
Release Date:
03/15/2016 - 4:30pm


