Cancers with a particularly poor prognosis pose a major challenge to health care in the 21st century. New research from Erik Knudsen, PhD, and Agnieszka Witkiewicz, MD, at the University of Arizona Health Sciences, shows that a highly personalized, patient-directed approach is necessary to improve treatment outcomes.
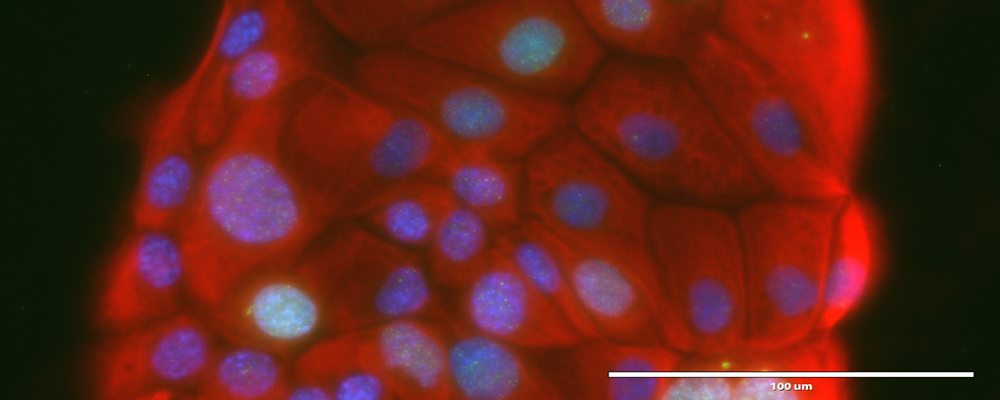
Advanced imaging and microscopic techniques that make cellular components visible help researchers tease apart the highly complex interactions of signaling pathways that govern the lives of cells, in both healthy and cancerous tissues. Understanding the various components of such interactions provides the basis for the development of more effective therapeutic approaches. Pictured here is patient-derived cell lines immunostained for CK8 (red), DAPI (blue) and H2AX (green). (Photo: Witkiewicz lab)

A team led by University of Arizona researchers is taking a new, patient-directed approach to treating pancreatic cancer.
Rather than relying on conventional cell lines that have defined effective drug targets for other types of cancers, they are creating and sequencing cell lines from a cancer patient's own tissue.
Drs. Agnieszka Witkiewicz and Erik Knudsen investigate tumors on the molecular level in hopes of opening up new approaches to more effective treatment. (Photo: BioCommunications, Kris Hanning)
Their results reveal that pancreatic tumors are more varied than previously thought and that drug sensitivity is unique to each patient, illustrating why recent efforts to boost "personalized medicine," such as the UA's Precision Medicine Initiative, are so important.
"Currently there are no targeted therapies directly against the hallmark mutations common in pancreatic cancer, and each patient-derived model we tested had its own unique therapeutic sensitivities," said Erik Knudsen, PhD, a member of the University of Arizona Cancer Center, professor of medicine at the UA College of Medicine - Tucson in the Division of Translational and Regenerative Medicine, and author of the study recently published in the journal Cell Reports. "I'd say that's why many pancreatic cancer clinical trials fail — it's that expectation that most tumors will respond in the same way to a drug."
"Pancreatic cancer is particularly challenging to treat," said corresponding author Agnieszka Witkiewicz, MD, a professor of pathology and medicine at the UA. "Since there are no early detection tests, the majority of patients present with advanced disease. By that time, the tumor has accumulated multiple genetic changes selecting for resistance to many therapies."
In the study, the team turned to a library of cancer drugs, representative of what's available to patients, and tested each individually against a panel of different cell lines: either conventional pancreatic cell lines, which often are used by researchers and pharmaceutical companies, or cell lines that the team developed directly from cancer patients.
While conventional pancreatic cell lines were more sensitive to standard drugs used in pancreatic cancer treatment, cell lines from patients were not, with only a few responding to any single-agent treatment.
A Highly Complex Cancer
Histological image of a patient-derived xenograft tumor. (Credit: Knudsen Lab) 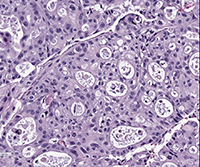
There are reasons why the response might have been so poor, Dr. Witkiewicz said. Typically, personalized medicine relies on genome sequencing, using a patient's tumor DNA to determine the mutations that cancer drugs can target. However, mutations that lead to cancer are complex, given that some can override others, making a singular mutation difficult to target. Combination therapy— using several different cancer treatments in conjunction — can solve this problem, but determining which drugs to use and in what doses requires models that replicate the genetics of the individual patient's tumor.
Development of cell lines directly from patients can be challenging. It's time consuming and requires appropriate authentication in relation to the tumor from which the cell lines were derived. Unfortunately, for most commonly used cell lines, the patient's tumor never was fully characterized genetically, and the cell lines were not monitored for changes over time. As shown in this study, these difficulties can be overcome, and new models that better reflect a patient's cancer can be developed.
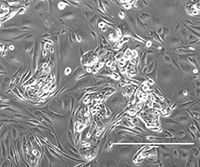 A phase contrast image of patient-derived cell lines early in the derivation process. (Credit: Knudsen Lab)
A phase contrast image of patient-derived cell lines early in the derivation process. (Credit: Knudsen Lab)
"There's a bit of frustration with the current personalized medicine approach," Dr. Knudsen said. "If you sequence a hundred tumors from patients in the clinic, you might be able to treat one or two patients with the resulting information, because of the nature of pancreatic cancer genetics. Using new, patient-derived models fills in the gap for us and lets us guide our therapies with functional sensitivities to drugs, not with preconceived notions."
Both researchers emphasized that testing the cell lines from greater numbers of patients is key in advancing pancreatic cancer treatment. Disease models that are derived from a single patient with the patient's specific mutations also are necessary to test the therapies derived from genomic sequencing before moving on to the patient. Both agreed that clinical trials that incorporate a drug-screened patient model will be critical for improving patients' outcomes.
UA Blazes Trail Toward Personalized Medicine
Efforts to understand the genetic and biological response of specific patient tumors already are underway at the UA, for example in the form of a tumor bank as part of the UA Cancer Center's Tissue Acquisition and Cellular/Molecular Analysis Shared Resource, or TACMASR, initiative, which is affiliated with the UA Biorepository through the UA Health Sciences.
 "We ask every patient undergoing surgery for permission to genetically characterize and better understand their tumor specimens through research," said Taylor Riall, MD, PhD, chief of the Division of General Surgery/Surgical Oncology in the UA Department of Surgery. "We find that most patients are excited at this opportunity. They understand their tissues are used for many different research projects that ultimately will benefit patients similar to themselves in Arizona and elsewhere."
"We ask every patient undergoing surgery for permission to genetically characterize and better understand their tumor specimens through research," said Taylor Riall, MD, PhD, chief of the Division of General Surgery/Surgical Oncology in the UA Department of Surgery. "We find that most patients are excited at this opportunity. They understand their tissues are used for many different research projects that ultimately will benefit patients similar to themselves in Arizona and elsewhere."
Riall, who was not involved in the published study, called the work "really exciting" and emphasized the need to look beyond genetics in personalized medicine.
"Anything that helps us physicians make rational therapy decisions that take into account the patient's individual situation is more likely to result in better outcomes," she said. "Being able to understand not only the genetic makeup of a given tumor, but also how it behaves in terms of its biology and response to therapy, helps us make better decisions for therapeutic options aimed at improving patient survival."
Through its Precision Medicine Initiative, the UA is at the forefront in the quest to find new ways of preventing and treating disease by taking advantage of differences among individuals as well as differences in the molecular fingerprint or abnormality of the diseased organ.
Recently the National Institutes of Health awarded the University of Arizona Health Sciences and Banner Health a $4 million research grant that will total more than $43 million over five years to participate in the national Precision Medicine Initiative® Cohort Program. UA-Banner is one of only four regional Healthcare Provider Organizations in the U.S. selected to spearhead a national cohort that eventually will include 1 million volunteer participants. As an HPO, the UA Health Sciences will help carry out unprecedented research projects to develop preventive and therapeutic strategies tailored to individualized care instead of one-size-fits-all health care.
'Right Treatment, Right Person, Right Time'
Under the PMI Cohort Program, the UA and Banner Health will recruit 150,000 or more participants, including patients as well as healthy individuals.
 "In addition to data about genetic makeup, lifestyle and environment, we will be collecting electronic health records as well as biological specimens that will help us create a platform for discoveries at the national level to realize the promises of personalized medicine," said Akinlolu Ojo, MD, MPH, PhD, vice president for clinical research and global health initiatives at the UA Health Sciences. "The central idea of precision medicine is to deliver the right treatment to the right person at the right time, and we believe that such a platform will make it possible in the very near future to develop an approach to prevention and treatment that is more effective, less expensive and safer."
"In addition to data about genetic makeup, lifestyle and environment, we will be collecting electronic health records as well as biological specimens that will help us create a platform for discoveries at the national level to realize the promises of personalized medicine," said Akinlolu Ojo, MD, MPH, PhD, vice president for clinical research and global health initiatives at the UA Health Sciences. "The central idea of precision medicine is to deliver the right treatment to the right person at the right time, and we believe that such a platform will make it possible in the very near future to develop an approach to prevention and treatment that is more effective, less expensive and safer."
According to Dr. Ojo, the research reported here has implications beyond very serious conditions such as pancreatic cancer.
"There are many other, common disorders for which treatment is more effective and less expensive when it's targeted based on unique information," he said, "whether that information is in the genetic architecture of an individual, their cells, their organs, or in the pathways involved in metabolizing drugs and handling them within that particular body.
"It's a holistic and individualized approach to health care," he added. "Cancer is an obvious target, but diseases of the cardiovascular system, the brain and the nervous system are important as well, as are inherited diseases and diseases affecting children."
"The path forward in studying pancreatic cancer is one that marries genetic analysis while also functionally analyzing drug sensitivities," Dr. Knudsen said. "This isn't a part of any conventional trial design in pancreatic cancer today."
"All of our work is about the patients at the end of the day — it's about a disease where standard approaches repeatedly have failed and patients really need hope," Dr. Witkiewicz added. "I think this work seeds new ideas for changing the paradigm for the treatment of pancreatic cancer — especially when there are so many failed trials. It will take a concerted effort from all of us, in academia, in pharma, in the clinic — everywhere."
A copy of the research article is available in the UA Campus Repository. The UA Campus Repository promotes open access by sharing and preserving research from the University of Arizona.
EXTRA INFO
Did you know?The National Pancreas Foundation recently accredited the UA College of Medicine – Tucson and Banner – University Medical Center Tucson as a Treatment Referral Center, one of 30 new such centers and the only one in Arizona. The designation recognizes a multidisciplinary team for its ability to handle complex care of patients suffering from pancreatitis, pancreatic cancer and related diseases. Read more…
ALSO SEE
For additional coverage of this story, check these:

